Enteroctopus dofleini (Wulker, 1910)Common name(s): Pacific giant octopus, Giant Pacific octopus, North Pacific giant octopus |
|
| Synonyms: Octopus dofleini, Octopus apollyon, Octopus hongkongensis, Polypus hongkongensis, Polypus apollyon, Paroctopus sp, Octopus punctatus |  |
| Phylum
Mollusca
Class Cephalopoda Order Octopoda Family Octopodidae |
|
| Enteroctopus dofleini in a seawater tank at Rosario. Arm spread about 1.5 meters | |
| (Photo by: Dave Cowles, July 2005) | |
How to Distinguish from Similar Species:Octopus rubescens is smaller, with mantle length less than 10 cm and weight less than 200 g; its skin has small, pointed papillae but not the large skin folds found on O. dofleini.
Geographical Range: Bering Sea to California; Northern Asia, Japan (and presumably Hong Kong)
Depth Range: Intertidal to 100 (180) m
Habitat: Primarily rocky subtidal; occasionally low intertidal or on sand
Biology/Natural History: The 3rd right arm of the male of this species has a large hectocotylus, about 1/5 the length of the arm (photo). The hectocotylus is used in transferring the male's spermatophore, or package of sperm, which may be up to a meter long, to the female. The hectocotylus may be left within the mantle of the female during the process. Eggs, which look like small whitish grapes, are laid throughout the year but mainly in the winter. When the female has eggs she attaches them to the roof of a cave and guards them until they hatch (5-7 months). She may lay 35,000 to 70,000 eggs in a single clutch. Hatching is mainly in early spring, and the young are pelagic for one to several months before settling. The young are sometime seen swimming near the surface. Lifespan is thought to be 4-5 years. Prey include crustaceans (shrimp and crabs), mollusks (scallops, clams, abalones, moon snails, small octopus), and fish (rockfish, flatfish, sculpins). The octopus are often captured in crab traps, where they are trying (successfully) to steal the crabs. Females can be cannibalistic. The Seattle Aquarium recently observed an octopus catching the spiny dogfish Squalus acanthias, and in 2005 we found the picked-clean skeleton of a dogfish on the shellheap outside an octopus den (photo). The species accumulates a large pile of shells and crab carapaces outside the den, which is usually under a boulder or in a rocky crevice. They quickly kill crabs by rasping a tiny hole (1 mm or less in diameter) through the carapace (photo), probably with their radula, then presumably injecting poison, perhaps with their beak. Several species may be attracted to their shell pile (midden), including Pycnopodia helianthoides and the snail Amphissa columbiana. Predators include seals, sea otters, dogfish sharks, lingcod, and man. Parasites include the mesozoans Dicyemenna abreida and Conocyema deca, which live in the kidney.
This octopus is said to be capable of inflicting a painful bite but I have never seen anyone bitten, even when wrestling them off the rocks. They seem much less ready to bite than is O. rubescens.
| Return to: | |||
| Main Page | Alphabetic Index | Systematic Index | Glossary |
References:
Dichotomous Keys:Jorgensen, 2009
Flora and Fairbanks, 1966 (As Octopus apollyon)
Kozloff 1987, 1996
Scott and Blake, 1998
General References:
Brusca
and Brusca, 1978
Gotshall,
1994
Johnson
and Snook, 1955 (as Polypus hongkongensis)
Kozloff,
1993
McConnaughey
and McConnaughey, 1985
Morris
et al., 1980
Norman, 2003
O'Clair
and O'Clair, 1998
Ricketts
et al., 1985
Sept,
1999
Scientific Articles:
Anderson, Roland C. and Daniel H. Blustein, 2006. Smart
octopus?
The Festivus 38:1 pp 7-9 (San Diego Shell Club)
Brocco, S. L. and R. A. Cloney, 1980. Reflector cells in the skin of Octopus dofleini. Cell Tissue Res. 205: 167-186
Dodge, R. and D. Scheel, 1999. Remains of the prey--recognizing the midden piles of Octopus dofleini (Wulker). The Veliger 42: 260-266
Fisher, W. K., 1923. Brooding habits of a cephalopod. Ann. Mag. Natural History, ser. 9, 12: 147-149
Gabe, S.H., 1975. Reproduction in the giant octopus of the North Pacific, Octopus dofleini martini. Veliger 18: 146-150
Hartwick, E.B. and G. Thorarinsson, 1978. Den associates of the giant Pacific octopus, Octopus dofleini (Wulker). Ophelia 17: 163-166
Hartwick, E. B., G. Thorarinsson and L. Tulloch, 1978. Antipredator behavior in Octopus dofleini (Wulker). Veliger 21: 263-264
Hartwick, E. B., G. Thorarinsson and L. Tulloch, 1978. Methods of attack by Octopus dofleini (Wulker) on captured bivalve and gastropod prey. Mar. Behav. Physiol. 5: 193-200
Hochberg, F.G., 1998. Class Cephalopoda: Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and the Western Santa Barbara Channel. Volume 8 part 1: The Aplacophora, Polyplacophora, Scaphopoda, Bivalvia and Cephalopoda, pp. 1-250. P.V. Scott and J.A. Blake, Editors. Santa Barbara Museum of Natural History, Santa Barbara, CA, USA
Mather, J.A. and R.C. Anderson, 1999. Exploration, play and habituation in octopuses (Octopus dofleini). J. Comparative Psychology 113:3 pp 333-338Mather, J.A., S. Resler, and J. Cosgrove, 1985. Activity and movement patterns of Octopus dofleini. Mar. Behav. Physi. 11: 301-314
Pickford, G.E., 1964. Octopus dofleini (Wulker), the giant octopus of the North Pacific. Bulletin of the Bingham Oceanographic Collection, Peabody Museum of Natural History, Yale University 19: 1-70
Rigby, P. Robin and Yasunori Sakurai, 2004. Temperature and feeding related growth efficiency of immature octopuses Enteroctopus dofleini. Suisanzoshoku 52:1 pp 29-36
Scheel, D., 2002. Characteristics of habitats used by Enteroctopus dofleini in Prince William Sound and Cook Inlet, Alaska. Mar. Ecol. 23: 185-206
Vincent, T.L.S., D. Scheel, and K.R. Hough, 1998. Some aspects of diet and foraging behavior of Octopus dofleini in its northernmost range. Marine Ecology 19:13-29
Student Reports:
Onthank, Kirt L., and Nicolas C. Marsh, 2005. Enteroctopus
dofleini midden composition and characteristics near
Deception Pass,
Washington
Onthank, Kirt L., Mayberry, Jon P., and Nicolas C. Marsh, 2005. Feeding ecology of the giant pacific octopus near Deception Pass, Washington
Web sites:
General Notes and Observations: Locations, abundances, unusual behaviors:
We have captured a large individual of this species from at least 100 m depth in the San Juan Channel. The largest I know of being seen at Rosario was one with about a 4 m span near Northwest Island.

The bottom arm in this view is the hectocotylus arm (right arm #3)
in an Enteroctopus dofleini male of about 50 cm arm
spread.
Arm #4 is shown above for comparison.
This view shows the hectocotylus arm (right) and a normal arm (left) of a male E. dofleini which is being dissected.

Here is a closeup of the suckers on an individual with about a 1m arm
spread.
This view of a dissected E. dofleini shows the salivary glands. The salivary glands of many octopus secrete poison which can be excreted through the salivary papilla in the mouth and used to subdue prey.

This dogfish (Squalus acanthias) skeleton was found
outside
an octopus den in 2005 by divers. Finders include Jon
Mayberry (L),
Kirt Onthank (middle), and Nick Marsh
 |
 |
The carapace was collected from a shell midden outside an active den.
In experiments in the Rosario seawater tanks in summer, 2005, students Jon Mayberry and Kirt Onthank found that E.. dofleini prefers crabs over scallops. Click here for a movie showing the octopus exiting from its burrow and choosing to grab a crab rather than a scallop that was presented to it.
More detailed results from research papers by Kit Onthank, Jon Mayberry, and Nichola Marsh, 2005: Althouth the E. dofleini they captured had a clear preference for crabs over bivalves, the contents of three middens at Coffin Rocks in Bowman Bay had more bivalves than crabs. There was also substantial variation among middens in the proportion of different prey species. This suggests that different individuals may have different preferences or hunting patterns, and that bivalves may be more available to octopus in this area than crabs are. They also found that about 50% of the crab carapaces had drill marks, as seen above, while very few bivalve shells had drill marks and almost no scallops did. The literature suggests that octopus try to pry bivalves open first, and only drill them if they cannot pry them open. The large size of E. dofleini may allow it to open most bivalves and nearly all scallops without drilling them. They also found that about 25% of the scallop shells were broken in a characteristic manner--with one of dorsal extensions or "wings" of the shell broken off one valve and attached to the other valve. The valves apparently broke while the octopus was attempting to pry them open.
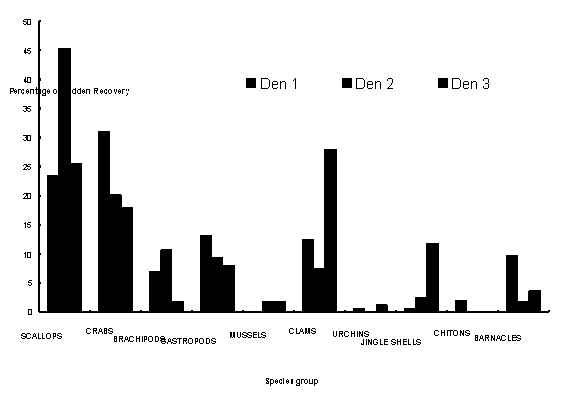
Figure 2 from Onthank et al., 2005: Percentage of
each prey type
from E. dofleini middens, by prey type
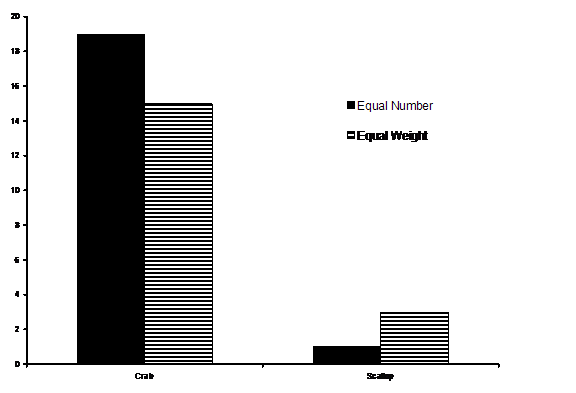
Figure 3 from Onthank et al., 2005: Result of E.
dofleini
feeding preference test, in which an octopus in an aquarium was
presented
with a choice between crabs (Cancer productus) and
scallops (Chlamys
hastata). The octopus clearly preferred crabs, even
though the
midden had more scallop shells.

This photo shows a baby, with total armspread under 10 cm, crawling
along the surface of a rock scallop.
Captured at 18 m sdepth outh of Keystone in drifting kelp.

Here the same individual shows off its tentacles on the side of the
aquarium.

Here the same individual has adjusted its chromatophores to blend in
with the surface of the shell it is upon.

This photo is a typical view of a very large individual within its
underwater burrow. Photo by Dave Cowles, July 2014
Authors and Editors of Page:
Dave Cowles (2005): Created original page
