Neognathophausia ingens (Dohrn, 1870)
Common name(s): Deep Water Giant Red Mysid, Giant luminescent
opossum shrimp (My name for it--this animal is rarely encountered at the
surface)
|
| Synonyms: Gnathophausia ingens,
Gnathophausia bengalensis, Gnathophausia calcarata, Gnathophausia doryphora,
Lophogaster ingens |
 |
Phylum Arthropoda
Subphylum Crustacea
Class Malacostraca
Subclass Eumalacostraca
Superorder Peracarida
Order Lophogastrida
Family Lophogastridae (or Gnathophausiidae) |
| A large sub-adult Gnathophausia ingens swimming past a 1-cm
grid. Note that swimming is accomplished with the pleopods,
which beat metachronously, and the left and right sides are in opposite
phase of beat. The thoracopods
are held tightly against the underside of the thorax. |
| (Photo by: Dave Cowles, 1987) |
Description: Lophogastrids are bathypelagic,
shrimplike crustaceans which differ from true shrimp in that their carapace
overhangs but is not actually connected to the posterior thoracic
segments. They are not decapods as shrimp are, and, for example,
have only one set of maxillipeds
instead of 3 and 7 pairs of pereopods
instead of 5. The pleopods,
with which they swim (see photo above), are well developed. They
have large thoracic
gills but no statocysts.
As a Peracaridan, female Lophogastrids have long thoracic
endopods (called oostegites) which are modified into a basket for carrying
eggs and larvae (photo). This species is
the largest pelagic crustacean. Maximum length up to 35 cm (for a
large female captured in the eastern tropical Pacific--Clarke, 1961).
Most are less than 18 cm long.
How to Distinguish from Similar Species: The
rostrum is shorter than that of Gnathophausia zoea and Neognathophausia
gigas and is indistinctly denticulate. Has reduced or no
supra-orbital spines. The spines at the posterolateral margin of
the carapace are also shorter than those of Gnathophausia zoea.
Unlike Gnathophausia gracilis,
this species does not have prominent dorsal spines on the abdominal segments.
Unlike Neognathophausia gigas,
both the anterior and the posterior lobe of the pleural plates are spiniform.
Geographical Range: Worldwide in tropical
and temperate seas, most common in tropical and subtropical zones (mainly
fron 40 degrees N to 40 degrees S). Common bathypelagically
off California and West Africa. Less common in the eastern tropical
Pacific and eastern tropical Atlantic than in the equatorial Indian Ocean,
probably because of the extremely low oxygen levels in the eastern tropical
Pacific at the depths G. ingens inhabits.
Depth Range: Usually around 500-900
m (brooding females often around 1200 m). Can be found down to 4000
m. Juveniles are usually in water from 5 to 8 degrees C.
Habitat: Bathypelagic
Biology/Natural History: Lophogastrids
were formerly thought to be a type of mysid. In regions where this
species is common, males do not reach the maximum size. After the
instar at which the species reaches sexual maturity (at about 15 cm total
length), the females undergo a growth spurt and the males seem to disappear
from the population. This implies that the females may eat the males
after copulation. Very large males seem to be found mainly in areas
where the species is scarce and the male may not have encountered and bred
with a female. While brooding eggs, the female sinks down to around
1000-1200 m depth. She carries the eggs and larvae for about 1.5
years, during which time she loses much organic body mass and is apparently
not feeding (Childress and Price, 1983). The female's eggs account
for 61% of the energy she has accumulated over her lifetime.
Another 13% is used during brooding of her young, 6% in cast exoskeletons,
and she only retains 20% of her original total energy content after brooding
(but her water content is very high). The species reproduces only
once, and the female dies shortly after release of the larvae. The
species has 13 instars. Intermolt interval varies from 166 to 253
days, depending on the size (Childress and Price, 1978.
This species lives permanently below the euphotic zone. Although
its water content increases in winter, suggesting fluctuations in food
availability, its O:N ratio changes little indicating that its lipid levels
remain high and it is not starving (Hiller-Adams and Childress, 1983).
Both its metabolic rate and ammonia excretion decrease with starvation
(Hiller-Adams and Childress 1983, Quetin et al., 1980).
Neognathophausia ingens swims primarily with the pleopods, with
some participation by the thoracic exopods (Hessler, 1985). Their
activity levels are little affected by pressure (Quetin and Childress,
1980). The species swims constantly and has a relatively high
drag compared to fish (Cowles et al., 1985), but swims at a speed which
minimizes energy losses due to drag (Cowles and Childress, 1988).
Gnathophausia means "light-jaw". This species has a gland
on its second maxillae (mouthparts) from which it spews a brilliantly luminescent
cloud into the water when disturbed. Luminescence seems to be a function
of diet, since animals maintained on non-luminescent food in the laboratory
gradually lose their ability to luminesce, while if luminescent food is
restored they can regain their luminescence (Frank et al., 1984).
This species often lives in oxygen minimum layers, yet its metabolism
is entirely aerobic (Childress 1968, 1969, 1971, Cowles et al., 1991).
To facilitate oxygen diffusion, it maintains a high rate of oxygen flow
over its gills and extracts a very high percentage of the available oxygen
(Childress, 1971). Its low rate of aerobic metabolism (Childress,
1971, Cowles, 1987, Cowles et al., 1991) help keep it from building up
oxygen debt. It has greater gill surface area than do most crustaceans
and fishes (Belman and Childress, 1976). The oxygen diffusion distance
across the gills is 1.5 to 2.5 microns, comparable to that found in many
fishes (Belman and Childress, 1976). It maintains relatively high
rates of blood flow via large circulatory system components. Its
heart rate is similar to that of other similarly-sized crustaceans, and
the heart slows as oxygen limitation is reached (Belman and Childress,
1976). It appears that much of the oxygen in the blood is carried
by hemocyanin, which has a high oxygen affinity and cooperativity
and a large Bohr shift (Sanders and Childress, 1990). Species which
live in areas with very low oxygen levels, such as off California, are
able to live aerobically at lower oxygen levels than are those from higher
oxygen levels such as Hawaii (Cowles et al., 1991).
Predators include the Melanostominid fish Echiostoma barbatum
(Sutton and Hopkins, 1996), the Macrourid fish Macrouronus novaezelandiae
(Clark, 1985), dwarf sperm whale (Cardona-Maldonado and Mignucci-Giannoni
(1999), the Antillean beaked whale (Debrot, 1998), in which it comprised
41% of the stomach contents of a beached individual, and Cuvier's beaked
whale (Palacios, 2003).
The rostrum and spines of small individuals are relatively longer than
in large individuals. This led to small individuals originally having
been named a separate species, Gnathophausia calcarata.
Gnathophausia ingens is sometimes parasitized by an ellobiopsid
flagellate protozoan, Amallocystis fascitus, which forms a cluster
of white filaments on the ventral side of the anterior abdominal segment.
The parasite seems to be associated with the main nerve ganglion in this
segment, and is associated with hypertrophy of the ganglion. It also
retards sexual maturation such as retarded development of oostegites in
females and feminizing changes in males.
References:
Dichotomous Keys:
Kathman, R.D., W.C. Austin, J.C. Saltman, and J.D. Fulton, 1986.
Identification manual to the Mysidacea and Euphausiacea of the Northeast
Pacific. Canadian Special Publication of Fisheries and Aquatic Sciences
93. ISBN 0-660-12096-8
Pequegnat, L.H., 1965. The bathypelagic mysid Gnathophausia
(Crustacea) and its distribution in the eastern Pacific Ocean. Pacific
Science 19: 399-422
General References:
Scientific Articles:
Belman BW, Childress JJ (1976) Circulatory adaptations to the oxygen
minimum layer in the bathypelagic mysid Gnathophausia ingens. Biol.Bull.
150:15-37
Benson AA, Lee RF (1975) The role of wax in oceanic food chains. Scientific
American 232:76-86
Blaxter, JHS, Russell FS, Yonge M, 1980. The species of mysids
and key to genera. Advances in Marine Biology 18: 6-342
Brandt,
A., Muhlenhardt-Siegel, U, Siegel, V (1998) An account of the
mysidacea (Crustacea: Malacostraca) of the southern ocean. Antarctic
Science 10(1) 3-11
Cardona-Maldonado,
Maria A. and Antonio A. Mignucci-Giannoni, 1999. Pygmy and dwarf
sperm whales in Puerto Rico and the Virgin Islands, with a review of Kogia
in the Caribbean. Caribbean J. Science 35:1-2 pp 29-37
Casanova JP, De Jong L, Faure E (1998) Interrelationships of the two
families constituting the lophogastrida (crustacea:Mysidacea) inferred
from morphological and molecular data. Marine Biology 132:59-65
Childress JJ, Nygaard M (1974) Chemical composition and buoyancy of
midwater crustaceans as a function of depth of occurrence off Southern
California. Marine Biology 27:225-238
Childress JJ, Price MH (1978) Growth rate of the bathypelagic crustacean
Gnathophausia
ingens (Mysidacea:Lophogastridae) I. Dimensional growth and population
structure. Marine Biology 50:47-62
Childress JJ, Price MH (1983) Growth rate of the bathypelagic crustacean
Gnathopausia
ingens (Mysidacea:Lophogastridae)II.Accumulation of material and energy.
Marine Biology 76:165-177
Childress JJ (1968) Oxygen minimum layer:vertical distribution and respiration
of the mysid Gnathophausia ingens. Science 160:
Childress JJ (1977) Physiological approaches to the biology of midwater
organisms. In: Andersen NR (ed) Oceanic Sound Scattering Prediction. Plenum
Press, New York, p 301-324
Childress JJ (1971) Respiratory adaptations to the oxygen minimum layer
in the bathypelagic mysid Gnathopausia ingens. Biol.Bull. 141:109-121
Childress JJ (1971) Respiratory rate and depth of occurrence of midwater
animals. Limnology and Oceanography 16:104-106
Childress JJ (1975) The respiratory rates of midwater crustaceans as
a function of depth of occurrence and relation to the oxygen minimum layer
off southern California. Comp.Biochem.Physiol. 50A:787-799
Childress JJ (1995) Trends in Ecology and Evolution. Elsevier Trends
Journals 10:30-36
Clark, Malcolm
R., (1985) The food and feeding of seven fish species from the
Campbell Plateau, New Zealand. New Zealand J. Marine and Freshwater
Research 19: pp 339-363
Clarke WD (1961) A giant specimen of Gnathophausia ingens (Dohren,1870)(Mysidea)
and remarks on the assymmetry of the paragnaths in the suborder lophogastrida.
Crustaceana 2:313-324
Cowles DL (1985) An unusual relationship found between swimming velocity
and drag in negatively buoyant pelagic crustaceans. 1985 AAAS Annual Meeting
Abstracts. American Association for the Advancement of Science, Washington,
DC, p 130
Cowles DL (1986) Metabolism of deep-living pelagic crustaceans in relation
to depth of occurrence and environmental oxygen levels. EOS, Transactions,
American Geophysical Union 67:971
Cowles, David L., 1987. Factors affecting the aerobic metabolism
of midwater crustaceans. Ph.D. dissertation, University of California,
Santa Barbara. 228 pp.
Cowles, David L., Childress, James J., 1988. Swimming speed and
oxygen consumption in the bathypelagic mysid Gnathophausia ingens.
Biological Bulletin 175: 111-121
Cowles DL, Childress JJ, Gluck DL (1986) New method reveals unexpected
relationship between velocity and drag in the bathypelagic mysid Gnathophausia
ingens. Deep-Sea Research 33:865-880
Cowles DL, Childress JJ, Wells ME (1991) Metabolic rates of midwater
crustaceans as a function of depth of occurrence off the Hawaiian Islands:
Food availability as a selective factor? Marine Biology 110:75-83
Debrot,
Adolphe, 1998. New cetacean records for Curacao, Netherlands
Antilles. Caribbean Journal of Science 34:1-2 pp. 168-169
DeJong, L. and J.P. Casanova, 1997. Comparative morphology of
the foregut of four Gnathophausia species (Crustacea; Mysidacea; Lophogastrida).
Relationships with other taxa. Journal of Natural History 31:7 pp.
1029-1040
Denton EJ, Gray J (1985) Lateral-line-like antennae of certain of the
Penaeidea (Crustacea,Decapoda,Natantia). Proc.R.Soc.Lond.B. 226:249-261
Donnelly J, Stickney DG, Torres JJ (1993) Proximate and elemental composition
and energy content of mesopelagic crustaceans from the eastern Gulf of
Mexico. Marine Biology 115:469-480
Frank TM, Widder EA, Latz MI, Case JF (1984) Dietary maintenance of
bioluminescence in a deep-sea mysid. Journal of Experimental Biology 109:385-389
Fuzessery ZM, Childress JJ (1975) Comparative chemosensitivity to amino
acids and their role in the feeding activity of bathypelagic and littoral
crustaceans. Biol.Bull. 149:522-538
Hessler RR (1985) Swimming in crustacea. Transactions of the Royal Society
of Edinburgh 76:115-122
Hiller-Adams P, Childress JJ (1983) Effects of prolonged starvation
on O{-2} consumption, NH{+4} excretion, and chemical composition of the
bathypelagic mysid Gnathophausia ingens. Marine Biology 77:119-127
Hiller-Adams P, Childress JJ (1983) Effects of season on the bathypelagic
mysid Gnathophausia ingens: water content, respiration, and excretion.
Deep-Sea Research 30:629-638
Hopkins TL, Flock ME, Gartner Jr JV, Torres JJ (1994) Structure and
trophic ecology of a low latitude midwater decapod and mysid assemblage.
Marine Ecology Progress Series 109:143-156
Kathman, R. D., W. C. Austin, J. C. Saltman, and J. D. Fulton (1986)
Identification Manual to the Mysidacea and Euphausiacea of the Northeast
Pacific. Canadian Special Publication of Fisheries and Aquatic Sciences,
vol. 93. 411 pp. Department of Fisheries and Oceans, Ottawa,
Canada
Mickel TJ, Childress JJ (1982) Effects of pressure and pressure acclimation
on activity and oxygen consumption in the bathypelagic mysid Gnathophaysia
ingens. Deep-Sea Research 29:1293-1301
Mickel TJ, Childress JJ (1978) The effect of pH on oxygen consumption
and activity in the bathypelagic mysid Gnathophausia ingens. Biol.Bull.
154:138-147
Moeller JF, Case JF (1994) Properties of visual interneurons in deep-sea
mysid,Gnathophausia ingens. Marine Biology 119:211-219
Moeller JF, Case JF (1995) Temporal adaptions in visual systems of deep-sea
crustaceans. Marine Biology 123:47-54
Ortmann AE (1906) Schizopod Crustaceans in the United States National
Museum- the Families Lophogastridae and Eucopiidae. Government Printing
Office, Washington DC
Palacios,
Daniel M., 2003. Oceanic Conditions Around the Galapagos Archipelago
and their influence on cetacean community structure. Ph.D. dissertation,
Oregon State University
Pequegnat, Linda H., 1965. The bathypelagic mysid Gnathophausia
(Crustacea) and its distribution in the eastern Pacific Ocean. Pacific
Science 19:4 399-421
Petryshov, V. V. (1992) Notes on mysid systematics (Crustacea,
Mysidacea) of Arctic and the north-western Pacific. Zoologichesky
Zhurnal 71:10 pp. 47-58 (In Russian, with English abstract)
(This is the article in which the species was assigned to the genus
Neognathophausia)
Quetin LB, Mickel TJ, Childress JJ (1978) A method for simultaneously
measuring the oxygen consumption and activity of pelagic crustaceans. Comp.Biochem.Physiol.
59A:263-266
Quetin LB, Ross RM, Uchio K (1980) Metabolic characteristics of midwater
zooplanton:ammonia excretion, O:N ratios, and the effect of starvation.
Marine Biology 59:201-209
Quetin LB, Childress JJ (1980) Observations on the swimming activity
of two bathypelagic mysid species maintained at high hydrostatic pressures.
Deep-Sea Research 27A:383-391
Quetin LB, Childress JJ (1981) Oxygen consumption of the bathypelagic
mysid Gnathophausia ingens as a function of swimming activity in
relation to oxygen and temperature.
Roe HSJ (1984) The diel migrations and distributions within a mesopelagic
community in the north east Atlantic. 2. Vertical migrations and feeding
of mysids and decapod crustacea. Progress in Oceanography 13:269-318
Sanders NK, Childress JJ (1990) Adaptations to the deep-sea oxygen minimum
layer: oxygen binding by the Hemocyanin of the Bathypelagic mysid, Gnathophausia
ingens Dohrn. Biol.Bull. 178:286-294
Tattersall WM (1951) A Review of the Mysidacea of the United States
National Museum. United States Government Printing Office, Washington,
DC
Web sites:
General Notes and Observations: Locations, abundances,
unusual behaviors:
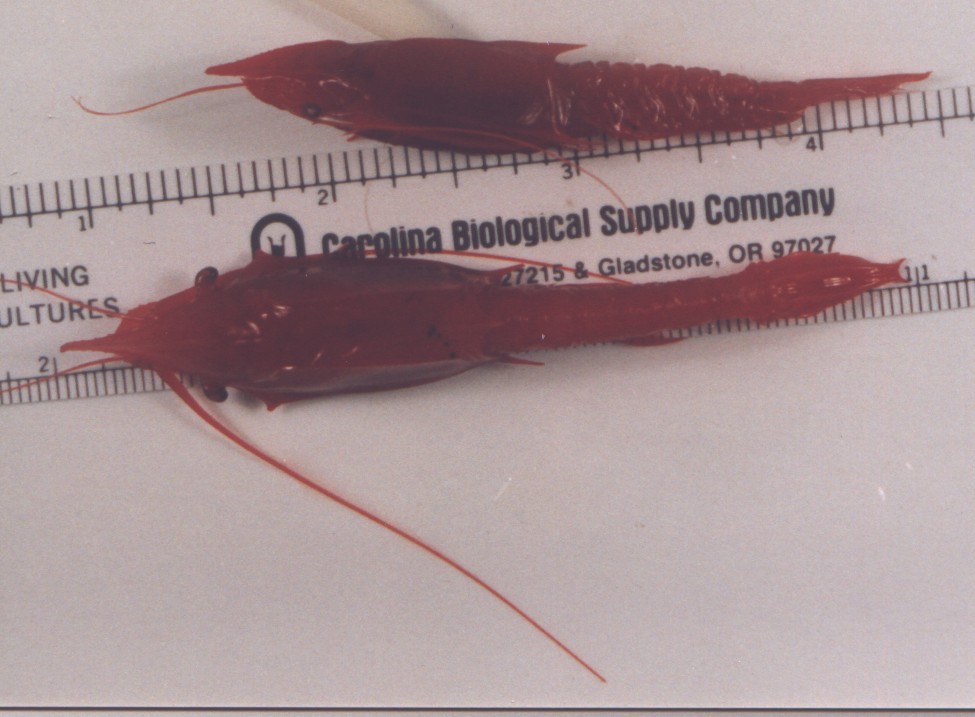
These juvenile individuals were captured in San Clemente Basin at about
700-800 m depth. Photo by Dave Cowles, May 1996
Note the two spines at the end of the telson which form a crescent-shaped
structure characteristic of Gnathophausia and Neognathophausia
Notice also that the rostrum in this juvenile is longer in proportion
to the animal's total length than is the rostrum of the adult female below,
but that it is shorter than that of Neognathophausia
gigas.

Mysids and Lophogastrids are "opossum shrimps" because the females
carry their eggs and young in a thoracic pouch or "marsupium". The
dorsal wall of the pouch is the ventral surface of the thorax,
while the ventral wall is composed of "oostegites", which are inner,
plate-like processes (endopods) projecting from the coxa of the thoracic
legs of mature females. The processes overlap one another, forming
the pouch. In this 15 cm female with young, the pouch can be clearly
seen. She is live and swimming with her pleopods. Photo by
Dave Cowles

Here is an even larger, live female with marsupium. Photo by
Dave Cowles, July 1983.
To view an mpg movie showing some of the key features used in identifying
Neognathophausia
ingens (15.8 Mb), click here.
Authors and Editors of Page:
Dave Cowles (2006): Created original page



