Upogebia pugettensis (Dana, 1852)Common name(s): Blue mud shrimp, marine crayfish |
|
| Synonyms: Gebia pugettensis, Gebia californica |  |
| Phylum Arthropoda
Subphylum Crustacea Class Malacostraca Subclass Eumalacostraca Superorder Eucarida Order Decapoda Suborder Pleocyemata Infraorder Thalassinidea Family Upogebiidae |
|
| Upogebia pugettensis from Padilla Bay. Total length approx. 8 cm. | |
| (Photo by: Dave Cowles, July 2005) | |
How to Distinguish from Similar Species: This is the only member of its family found near Rosario. Neotrypaea (formerly Callianassa) californiensis is the local species which looks most similar, but Neotrypaea has a non-hairy rostrum of one small tooth and chelate chelipeds which are decidedly unequal in size. Up to 150 mm long. See also the biology descriptions for how to distinguish their burrows.
Geographical Range: Valdez Narrows, Alaska to Morro Bay, CA (S of Morro Bay a nearly identical species, U. macginitieorum is found)
Depth Range: Mostly intertidal
Habitat: Mud flats
Biology/Natural History: This species lives in pairs in permanent, U- or Y-shaped burrows in mud flats, usually lower in the intertidal and in muddier areas than does Neotrypaea. The burrows are often 1 m or more deep, usually have several entrances, and may connect horizontally to the burrow of nearby mud shrimp. For burrowing they carry mud in a "basket" formed by the first two pairs of thoracic legs. The pelagic larvae preferentially settle in areas where adult burrows are present (Dumbauld et al., 2011). Once removed from the burrow as adults they do not seem to be able to dig another one. They feed on suspended detritus carried into their burrow by the beating of their pleopods and captured by their hairy first two pairs of thoracic legs. The fifth pair of legs is used both for walking (with legs 3-4) and for cleaning the body (plus the eggs of females). Many commensals such as the northern hooded shrimp Betaeus harrimani, the snapping shrimps Betaeus ensenadensis and B. longidactylus, the crab Scleroplax granulata, the pea crabs Pinnixa franciscana and P. schmitti, the scale worm Hesperonoe complanata, the clam Cryptomya californica, the phoronid Phoronis pallida, and the arrow goby Clevelandia ios live in the burrows. Upogebia also seems to have many parasites including an isopod which frequently lives in the gill chambers of specimens from Padilla Bay, the clam Pseudopythina (formerly Orobitella) rugifera which lives attached to the pleopods (picture), and the isopod Phyllodurus abdominalis, which also attaches to the pleopods. The copepod Clausidium vancouverense lives on the outside of the body. Upogebia lays yellow eggs in fall which do not hatch until spring. Predators include Pacific staghorn sculpin Leptocottus armatus. Mud shrimp disrupt commercial oyster beds and smother the oysters. This species can tolerate brackish water down to 10% of seawater salinity, by osmoregulation.
In an earlier study in Oregon's Yaquina Bay where Upogebia pugettensis is very abundant, 57% of males and 80% of females were infested with the parasitic, blood-sucking bopyrid isopod Orthione griffinis (Smith et al., 2008) Infestation was especially prevalent among larger Upogebia and in females. Infested hosts were an average of 7.8% lower weight than similar-sized uninfested hosts. Burrowing by the species increased remineralization of carbon compounds in the sediment by up to 2.9 times, ammonification by up to 7 times, nitrification by 3 to 9 times, denitrification by up to 4 times, and up to a 15-fold increase in flux of dissolved inorganic nitrogen.
During the early 2000's (2000-2014), Orthione griffinis has continued to spread and infest populations. This infestation is associated with sharp population declines and even local extinctions of Upogebia pugettensis (Chapman and Carter, 2014). All populations between Vancouver, BC and Morro Bay, CA, appear to be declining (Chapman et al., 2012). I have certainly observed that phenomenon in the Padilla Bay region near our Rosario Marine Laboratory. Within a few years of the appearance of O. griffinis, Upogebia pugettensis disappeared. I can no longer find them at all in areas where they were formerly abundant. Orthione griffinis effectively castrates the female Upogebia pugettensis (Chapman et al., 2012). They only parasitize Upogebia pugettensis that are larger than 12 mm carapace length (Smith et al., 2008; Dumbauld et al., 2011; Chapman et al., 2012).
Burrow openings of Upogebia pugettensis are Y-shaped and have two narrow openings at the surface of the mud. These two openings are almost always connected to one another. The walls of the burrow are quite smooth. These characteristics can be used to distinguish Upogebia pugettensis burrows from those of Neotrypaea californiensis, which typically builds burrows with multiple connected openings that do not constrict at the surface, and the walls of which are more rough. The clam Mya arenaria also lives in similar areas, and their siphons create smooth openings in the substrate similar to those of Upogebia burrows. However, Mya arenaria holes do not come in pairs and they are more oval in shape (Chapman and Carter, 2014).
| Return to: | |||
| Main Page | Alphabetic Index | Systematic Index | Glossary |
References:
Dichotomous Keys:Carlton, 2007
Flora and Fairbanks, 1966
Hart, 1982
Kozloff 1987, 1996
Smith and Carlton, 1975
Wicksten, 2009
General References:
Brusca
and Brusca, 1978
Harbo,
1999
Johnson
and Snook, 1955
Morris
et al., 1980
Niesen,
1994
Niesen,
1997
O'Clair
and O'Clair, 1997
Ricketts
et al., 1985
Sept,
1999
Scientific Articles:
Chapman, John W., and Cameron S. Carter, 2014. A rapid intertidal megafauna survey method applied to Upogebia pugettensis, and its introduced parasite, Orthione griffensis. Journal of Crustacean Biology 34:3 pp 349-356
Chapman, John W., BrettR. Dumbauld, Gyo Itani, and John C. Markham, 2012. An introduced Asian parasite threatens northeastern Pacific estuarine ecosystems. Biological Invasions 14: pp 1221-1236
D'Andrea, Anthony F. and Theodore H. DeWitt, 2009. Geochemical ecosystem engineering by the mud shrimp Upogebia pugettensis (Crustacea: Thalassinidae) in Yaquina Bay, Oregon: Density-dependent effects on organic matter reminaralization and nutrient cycling. Limnology and Oceanography 54:6 pp. 1911-1932
Dumbauld, B.R., D. A. Armstrong, and K. L. Feldman, 1996. Life-history characteristics of two sympatric thalassinidean shrimps, Neotrypaea californiensis and Upogebia pugettensis, with implications for oyster culture. Journal of Crustacean Biology 16: 689-708
Dumbauld, Brett R., John W. Chapman, Mark E. Torchin, and Armand M. Kuris, 2011. Is the collapse of mud shrimp (Upogebia pugettensis) populations along the Pacific coast of North America caused by outbreaks of a previously unknown bopytid isopod parasite (Orthione griffensis)? Estuaries and Coasts 34: pp 336-350
Griffen, B. D., T. H. DeWitt, and C. Langdon, 2004. Particle removal rates by the mud shrimp Upogebia pugettensis, its burrow, and a commensal clam: effects on estuarine phytoplankton abundance. Marine Ecology Progress Series 269: 223-236
Grundset, Edgar Olaf, 1959. Observations on the natural history of the blue mud shrimp Upogebia pugettensis (Dana). Masters Thesis, Walla Walla College, 1959. 100 pages.
Markham, John C., 2004. New species and records of Bopyridae (Crustacea: Isopoda) infesting species of the genus Upogebia (Crustacea: Decapoda: Upogebiidae): the genera Orthione Markham, 1988, and Gyge Cornalia & Panceri, 1861. Proceedings of the Biological Society of Washington 117(2): 186-198 (describes an isopod parasite, Orthione griffenis, from the gills of Upogebia in Oregon)
Posey, M. H., B. R. Dumbauld, and D. A. Armstrong, 1991. Effects of a burrowing mud shrimp, Upogebia pugettensis (Dana) on abundances of macro-fauna. Journal of Experimental Marine Biology and Ecology 148: 283-294
Smith, Andrew E., John W. Chapman, and Brett R. Dumbauld,
2008.
Population structure and energetics of the bopyrid isopod parasite Orthione
griffinis in mud shrimp Upogebia pugettensis.
Journal
of Crustacean Biology 28(2): 228-233
General Notes and Observations: Locations, abundances, unusual behaviors:
By a few years after the parasitic isopod Orthione griffenis appeared in the Padilla Bay/March Point population that we observe, the population had virtually disappeared. I saw not a single mud shrimp in the area for about 5 years. In 2015, however, we found one mud shrimp near its burrow. It was not parasitized by an isopod, but did have the symbiotic clam Pseudopythina (Orobitella) rugifera clinging to its pleopods.

The rostrum is triangular, has three teeth, and is covered with setae (which mostly obscure the teeth)
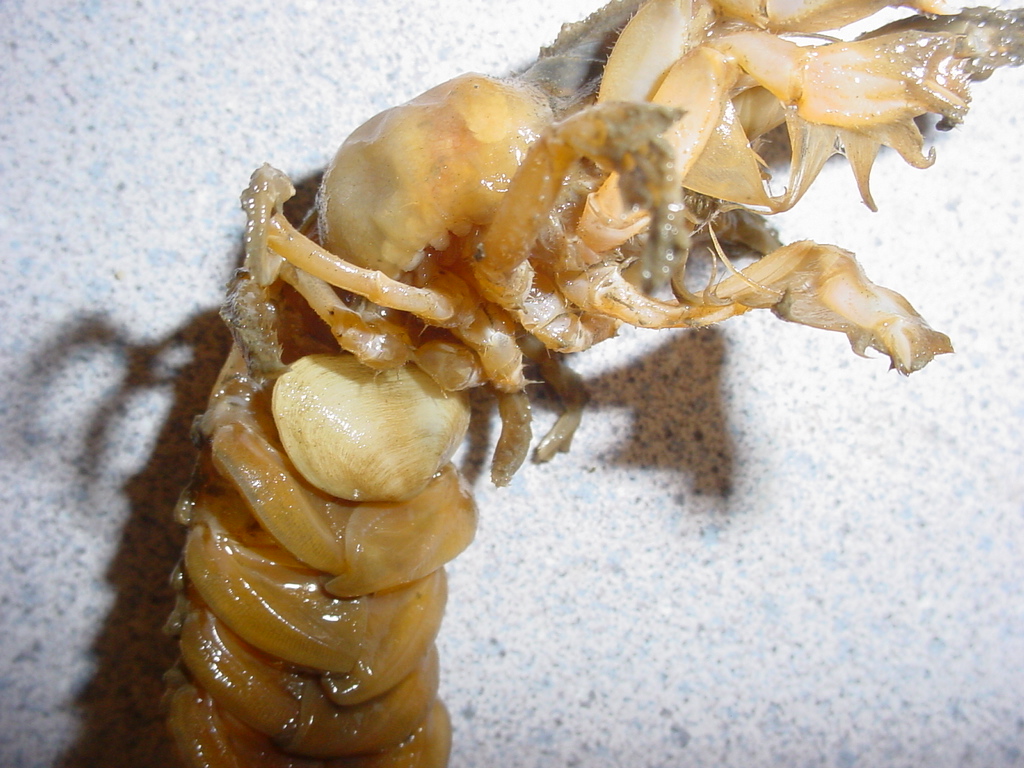
Upogebia is often parasitized by a clam, Pseudopythina
(Orobitella) rugifera, which clings to a
ventral part, usually
a pleopod. It is said to attach by a byssal thread.
An unknown parasitic isopod is living in the right gill chamber of
this specimen. Markham (2004) newly described a parasitic
isopod
Orthione
griffenis Markham, 2004 which lives in the gill chambers of
Upogebia,
so perhaps that is the species name here. Photo by Dave
Cowles, July 2005
Authors and Editors of Page:
Dave Cowles (2005): Created original page


