Metacarcinus magister (Dana, 1852) Schweitzer and Feldmann, 2000Common Name: Dungeness Crab, Market Crab, Common Edible Crab |
|
| Synonyms: Cancer magister |   |
| Phylum Arthropoda
Subphylum Crustacea Class Malacostraca Subclass Eumalacostraca Superorder Eucarida Order Decapoda Suborder Pleocyemata Infraorder Brachyura Superfamily Cancroidea Family Cancridae |
|
| Figure 1. Top and bottom view of Metacarcinus magister. Approximately 20 cm carapace width. Collected at Padilla Bay, WA. | |
| Photo by: Janisse Maxwell, taken 6-02. | |
How to Distinguish
from
Similar Species:
The most similar-appearing local species is Metacarcinus
gracilis, which has similar coloration
and white claw tips
as does this species. However, it has a distinct tooth behind
the
widest point of the carapace
and has no spiny ridges on the carpus,
propodus,
and dactyl
of the chelae.
It also does not grow as large.
Cancer
productus, also often found intertidally and
subtidally in the
Pacific Northwest, has black tips to the dactyls
of the chelae.
Note: Species formerly in genus Cancer have been recently
subdivided into several genera (Ng
et al., 2008; Schweitzer
and Feldmann, 2010). Of our local genera, Cancer, Romaleon,
and Metacarcinus
have a carapace wider than long plus only scattered setae on the
carapace margins and legs while Glebocarcinus
has a carapace of approximately equal length and width, often with
granular regions and with setae along the edges; and setae on the outer
surface of the chela as well as on the legs. Metacarcinus can be
distinguished from Cancer
because Metacarcinus
has anterolateral carapace teeth which are distinct and sharp plus the
male has a rounded tip to the telson, while Cancer
has anterolateral carapace teeth which are low and lobed, separated by
deep fissures plus the male has a sharply pointed telson (Schram
and Ng, 2012). Romaleon
can be distinguished from Cancer
and Metacarcinus
because it has a distinct tooth on the anterior third of the
posterolateral margin of the carapace while the other two genera do
not.
Geographical Range: Occurs from Alaska to Santa Barbara, California.
Depth Range: Lives from intertidal to a depth of 230 m.
Habitat: Most common in sand or muddy-sand bottoms in subtidal regions, often in or near eelgrass beds. Often partly buries itself in the sand.
Biology/Natural History: This crab is the largest edible crab from Alaska to California, making this species important for fisheries commercially and economically. There appear to be five subspecies in California alone. The female Dungeness crab can lay up to 2.5 million eggs and can live up to at least 6 years. Females can store sperm received during one mating season and use it during the next season. This species is a carnivore that feeds on more than 40 different species including small clams, oysters, fish, shrimp, worms and according to recent studies even feeds on Velella velella nematocysts. The larvae of this species is often attached to the bells of jelly fishes and to their tentacles; these larvae feed on the gonozooids, and by doing so gain protection from pelagic fish predators and are transported to juvenile crab habitats nearshore as long as associated with the cnidarian. Dungeness crab larvae feed primarily on zooplankton, however phytoplankton are also eaten. The larvae are crepuscular migrators, being found near the surface at dawn and dusk but deeper in midday and midnight. The stage 1 zoeae are nearest the surface with later zoeal stages in deeper water. In spring, larvae of this species may be advected north along the coast as far as Alaska (Park et al., 2007). In springtime, adults of this crab can be found buried in sand or in tidepools, where it can hide and wait for its new shell to harden. On average, males will cover more ground in an hour than females, and ovigerous females move less than nonovigerous females or males. Near Vancouver Island, adults have more epibionts than do juveniles (McGraw, 2006). Common epibionts include barnacles (especially Balanus crenatus) on the dorsal surface, green, red, and brown algae (especially on the antennae), tube-dwelling polychaetes (mainly on the ventral surfaces), hydrozoans (mainly on ventral surfaces and limbs), bryozoans (especially Membranipora membranacea) on any region of the carapace. A few had sponge, tunicate, or mollusk epibionts.
Feeding in ovigerous females is greatly reduced below that of non-ovigerous females. Females are able to survive an entire winter without feeding, at least in the laboratory. Both juvenile and adult crabs may sometimes be cannibalistic.
Dudas et al. (2005) found that the common local cancer crabs Metacarcinus magister and Cancer productus (red rock crab) preferred the thin-shelled introduced varnish clam Nuttallia obscurata to the thicker-shelled clams Leukoma staminea and Venerupis philippinarum if access to all was equally easy. However, Nuttallia obscurata typically lives deeper in the sediment than do Leukoma staminea or Venerupis philippinarum. If they had to dig for them, Metacarcinus magister still ate more Nuttallia obscurata than it did of the other clam species, but C. productus' preference switched to Leukoma staminea and Venerupis philippinarum.
Jensen and Bentzen (2012) found that the egg clutches of females frequently have multiple paternity. Adult females molt once a year and mate with one male per molt. They can store sperm for up to 2.5 years.
The hoplonemertean worm Carcinonemertes errans, an ectosymbiont and egg parasite in Metacarcinus magister, is in turn eaten by a Riserius sp nemertean whose larvae have previously been classified as pilidium recurvatum (Hiebert et al., 2013)
| Return to: | |||
| Main Page | Alphabetic Index | Systematic Index | Glossary |
References:
Dichotomous Keys:
Coffin, 1952
Flora and Fairbanks, 1966
Hart, 1982
Kozloff, 1987, 1996
Smith and Carlton, 1975
Wicksten, 2009
General References:
Kozloff,
1993.
Morris
et al., 1992.
O'Clair and O'Clair, 1998
Sept,
1999.
Scientific Articles:
Airriess, C., and McMahon, B., 1994. Cardiovascular Adaptations Enhance Tolerance of Environmental Hypoxia in the crab Cancer magister. Journal of Experimental Biology 190: 23-41
Bernatis, J., Gerstenberger, S., McGaw, I.,
2007.
Behavioural
responses of the Dungeness crab, Cancer magister,
during feeding
and digestion in hypoxic conditions. Marine Biology, Vol. 150, pp.
941-951
Burnett, Nicole, 2024: A practical identification guide to the zoeae of the invasive European green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda: Brachyura: Carcinidae), and to the zoeae of the families of brachyuran crabs in Washington state, USA. Journal of Crustacean Biology 44:4. doi.org/10.1093/jcbiol/ruae064
Curtis, D. L. and I. J. McGaw, 2008. A year in the life of the Dungeness crab: methodology for determining microhabitat conditions experienced by large decapod crustaceans in estuaries. Journal of Zoology 274:4 pp. 375-385
Curtis, Daniel L. and Iain J. McGaw, 2011. A possible feeding control mechanism in Dungeness crabs during hyposaline exposure. Journal of Crustacean Biology 31:2 pp. 313-316
Dudas, Sarah E., Iain J. McGaw, and John F. Dower, 2005. Selective crab predation on native and introduced bivalves in British Columbia. Journal of Experimental Marine Biology and Ecology 325:1 pp 8-17
Forward, Richard B. Jr., Richard A. Tankersley, and Dan Rittschof, 2001. Cues for metamorphosis of Brachyuran cabs: an overview. American Zoologist 41:5 pp. 1108-1122 (As Cancer magister)
Graham, R., 1985. A model for L-lactate binding to Cancer magister hemocyanin. Comp . Biochem. Phys., Vol. 81, pp. 885-887
Hankin, D.G., N. Diamond, M.S. Mohr, and J. Ianelli, 1989. Growth and reproductive dynamics of adult female Dungeness crabs, Cancer magister in northern California. Journal du Conseil Permanent International pour l'Exploration de la Mer 46: 94-108
Hobbs, R.C. and L.W. Botsford, 1992. Diel vertical migration and timing of metamorphosis of larvae of the Dungeness crab, Cancer magister. Marine Biology 112: 417-428
Jensen, Pamela C. and Paul Bentzen, 2012. A molecular dissection of the mating system of the Dungeness crab, Metacarcinus magister (Brachyura: Cancridae). Journal of Crustacean Biology 32:3 pp 443-456
Jensen, P.C., J.M. Orensanz, and D. Armstrong, 1996. Structure of the female reproductive tract in the Dungeness crab (Cancer magister) and implications for the mating system. Biological Bulletin 190: 336-349
Lough, R.G., 1976. Larval dynamics of the Dungeness crab, Cancer magister off the central Oregon coast, 1970-71. Fish. Bull. 74: 353-375
Losey, Robert J., Sylvia Behrens Yamada, and Leah Largaespada, 2004. Late-Holocene Dungeness crab (Cancer magister) harvest at an Oregon coast estuary. Journal of Archaeological Science 31: pp. 1603-1612
McConnaughey, R.A., D.A. Armstrong, B.M. Hickey, and D.R. Gunderson, 1994. Interannual variability in coastal Washington Dungeness crab (Cancer magister) populations: Larval advection and the coastal landing strip. Fish. Oceanogr. 3: 22-38
McGaw, I., 2005. Burying behavior of two sympatric crab species: Cancer magister and Cancer productus. Scientia Marina, Vol 69, pp. 375-381
McGraw,
Iain J., 2006. Epibionts of sympatric species of Cancer
crabs in Barkley Sound, British Columbia. J. Crustacean
Biology 26:1
85-93
Ng,
P.K.L., D. Guinot, and P.J.F. Davie, 2008.
Systema Brachyurorum: part I. An annotated
checklist of extant
brachyuran crabs of the world. Raffles Bulletin of Zoology,
Supplement
17 pp. 1-286 (Clicking on link will load a pdf of the long
article)Ng,
P.K.L., D. Guinot, and P.J.F. Davie, 2008.
Systema Brachyurorum: part I. An annotated
checklist of extant
brachyuran crabs of the world. Raffles Bulletin of Zoology,
Supplement
17 pp. 1-286 (Clicking on link will load a pdf of the long
article)
Park, Wongyu, David C. Douglas, and Thomas C. Shirley, 2007. North to Alaska: Evidence for conveyor belt transport of Dungeness crab larvae along the west coast of the United States and Canada. Limnology and Oceanography 52:1 248-256
Schram,
Frederick R. and Peter K.L. Ng, 2012. What is Cancer?
Journal of Crustacean Biology 32:4 pp. 665-672
Shirley, T.C. and L. McNutt, 1989. Precocious molting and
trans-molt
sperm retention by female Dungeness crabs. American Zoologist
29:
131A
Stillman, Jonathon H., John K. Colbourne, Carol E. Lee, Nipam H. Patel, Michelle R. Phillips, David W. Towle, Brian D. Eads, Greg W. Gelembuik, Raymond P. Henry, Eric A. Johnson, Michael E. Pfrender, and Nora B. Terwilliger, 2008. Advances in crustacean genomics. Integrative and Comparative Biology 48:6 pp 852-868
Thomton, Jamie D., Sherry L. Tamone, and Shannon Atkinson, 2006. Circulating ecdysteroid concentrations in Alaskan dungeness crab (Cancer magister). Journal of Crustacean Biology 26:2 pp 176-181
Sulkin, S. and G.L. McKeen, 1989. Laboratory
study
of survival
and duration of individual zoeal stages as a function of temperature in
the brachyuran crab Cancer magister.
Marine Biology 103: 31-37
General Notes and Observations: Locations, abundances, unusual behaviors, etc.:
Distinguishing Characteristics of Metacarcinus
magister:

The anterolateral carapace
margin has 10 teeth lateral to the eyes. The carapace
is widest at the 10th tooth, which is large. There are no
large teeth
on the posterolateral
margin of the carapace.

The chelipeds
are tipped with white. The carpus,
(on left in this photo) propodus
(middle in this photo), and dactyl
(right, movable finger of claw in this photo) have spiny ridges,
especially
along the anterior margin of the propodus
and dactyl.

The underside of the carapace
has hairlike setae
near the legs

The rear legs are flattened and are lined with setae
along some edges. This is the left rear leg.

Another photo of Metacarcinus magister, by Dave
Cowles

The many epibiont
barnacles encrusting this individual, found in a tidepool at Kalaloch
in
2009, implies that it has been some time since the animal has
molted.
These crabs spend a significant amount of time buried in the sand with
only their face projecting. This fact can be seen in this
individual
by noting that the barnacles do not encrust the back end, which is
usually
buried and without access to oxygen or food, but do encrust the front
end
which projects from the sand. Photo by Dave Cowles, July 2009

Regardless of how it may look, this is NOT a crab! See below for why.
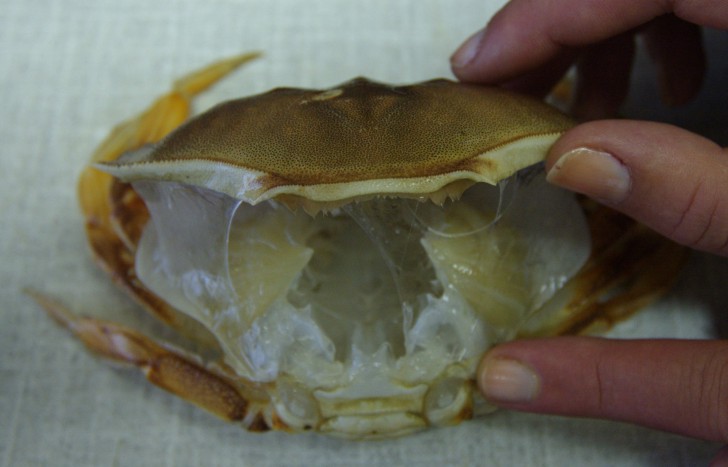
Even though the exoskeleton looks perfect, the occupant has backed out and left! This is just a molt or exuvium. Crabs molt the entire exoskeleton, including the lining of the gills (visible above), parts of the gut lining, the covering of the eyes, etc.

Crabs are quite vulnerable when they molt because not only are they struggling to get out of their old exuvium but their new exoskeleton is extremely soft and vulnerable. This crab died halfway through a molt because it was attacked by another crab when it was defenseless. Note the soft folds in the new exoskeleton, which is as soft as warm butter..
| Comparing the anatomy of male and female Dungeness crabs: | |
 |
 |
| The female abdomen is broad and covers most of the underside of the thorax between the leg bases. | The male abdomen is narrower than the female abdomen, comes to a sharper point, and does not cover most of the underside of the thorax between the leg bases. |
 |
 |
| The female abdomen has several sets of pleopods which are used for carrying the eggs. Note also her gonopores, which are on the underside of the 6th thoracic segment as is characteristic of decapods. | The male abdomen, in contrast, has only two sets of pleopods (on the two basal segments), which are used for transferring sperm. The male also has its gonopores on the 8th thoracic segment rather than on the 6th. |

Here is another photo of the underside of a male.
Authors and Editors of Page:
Janisse Maxwell (2002): Created original page..
Edited by Dave Cowles 2002, 2005, 2006, 2010, 2011
Edited by Hans Helmstetler 10-2002