Strongylocentrotus franciscanus (A. Agassiz, 1863)Common name(s): Red urchin, Giant red sea urchin |
|
| Synonyms: |  |
| Phylum Echinodermata
Class Echinoidea Subclass Euechinoidea Order Echinoida Family Strongylocentrotidae |
|
| Strongylocentrotus franciscanus collected from Sares Head, 15 m depth. Total width approximately 20 cm. Note the different colors of the two individuals. The two smaller urchins below are S. droebachiensis and S. purpuratus. | |
| (Photo by: Dave Cowles, August 1997) | |
How to Distinguish from Similar Species: Strongylocentrotus purpuratus is smaller and a strong purple color. Small S. franciscanus can look very much like S. purpuratus but S. franciscanus has longer spines--nearly as long as the test is wide. This species is common both on the open coast and protected waters, while S. purpuratus is found mostly on the open coast.
Geographical Range: Gulf of Alaska to Isla Cedros, Baja California; northern Japan
Depth Range: Low intertidal to 284 m; mostly subtidal.
Habitat: Rocky reefs, especially around kelp.
Biology/Natural History: Feed primarily on kelp (especially Nereocystis or Macrocystis) but can eat sessile invertebrates. Often forms large subtidal aggregations in or near kelp beds. A prime food for sea otters. Other predators include the sunflower star Pycnopodia helianthoides, leather star Dermasterias imbricata, red rock crab Cancer productus, spiny lobster Panulirus interruptus and sheephead fish (in S California), and humans. Commensals include the rhabdocoele flatworm Syndesmis (or Syndisyrinx) franciscanus, less than 1 cm long and lives inside the test, the isopod Colidotea restrata, which clings to the spines, and the amphipod Dulichia rhabdoplastis which builds rods of its fecal pellets that extend out from the urchin's spines (photo). The amphipod feeds on diatoms, which it seems to "farm" on the spines. Plots from which urchins were excluded became overgrown by large algae. Small urchins (less than 5 cm test diameter) often hide under the adults. In Puget Sound they spawn spring and summer. Pelagic echinopluteus larvae metamorphose into benthic juveniles after about 6-10 weeks. Young urchins often are found under larger individuals. Nishizaki and Ackerman (see ref below) found that adult urchins release a chemical cue that causes the young to aggregate underneath them when the adults detect the presence of Pycnopodia helianthoides. Ebert (1998) and Ebert and Southon (2003) determined that these urchins live over 100 years, and found some near Vancouver Island that may be 200 years old. They seem to reproduce best when in dense aggregations (Ebert 1998). Aggregations from which smaller individuals have been harvested recover much faster than those from which larger individuals have been removed (Rogers-Bennet et al., 1998). At depths over 70 m in the Salish Sea area they aggregate on shallow-slope bottoms near deposits of drift kelp, on which they feed (Britton-Simmons et al., 2012)
In September 2018, a team operating an OceanGate submersible commissioned by SeaDoc Society observed a red urchin and drift kelp at 284 m depth near the San Juan Islands, which more than doubles the known depth range for this species.
| Return to: | |||
| Main Page | Alphabetic Index | Systematic Index | Glossary |
References:
Dichotomous Keys:Flora and Fairbanks, 1966
Kozloff 1987, 1996
Smith and Carlton, 1975
General References:
Gotshall
and Laurent, 1979
Kozloff,
1993
Lambert
and Austin, 2007
O'Clair
and O'Clair, 1998
Scientific Articles:
Breen, P.A., W. Carolsfield, and K.L. Yamanaka, 1985. Social behaviour of juvenile red sea urchins, Strongylocentrotus franciscanus (Agassiz). Journal of Experimental Marine Biology and Ecology 92: 45-61
Britton-Simmons, Kevin H., Alison L. Rhoades, Robert E. Pacunski, Aaron W.E. Galloway, Alexander T. Lowe, Elizabeth A. Sosik, Megan N. Dethier, and David O. Duggins, 2012. Habitat and bathymetry influence the landscape-scale distribution and abundance of drift macrophytes and associated invertebrates. Limnology and Oceanography 57:1 pp. 176-184
Ebert, Thomas A., 1998. An analysis of the importance of Allee effects in management of the red sea urchin Strongylocentrotus franciscanus. pp. 619-627 in Rich Mooi and Malcolm Telford (eds), Echinoderms: San Francisco. Proceedings of the Ninth International Echinoderm Conference, San Francisco, California USA 5-9 August 1996.
Elbert, T.A., S.C. Schroeter, J.D.Dixon, and P. Kalvass, 1994. Settlement patterns of red and purple sea urchins (Strongylocentrotus franciscanus and S. purpuratus) in California, USA. Marine Ecology Progress Series 111: 41-52
Ebert, Thomas A., and John R. Southon, 2003. Red sea urchins (Strongylocentrotus franciscanus) can live over 100 years: confirmation with A-bomb 14carbon. U.S. Fishery Bulletin 101(4): 915-922
Levitan, D.R., M.A. Sewell, and F-S. Chia, 1992. How distribution and abundance influence fertilization success in the sea urchin Strongylocentrotus franciscanus. Ecology 73: 248-254
McCloskey, L.R., 1970. A new species of Dulichia (Amphipoda, Podoceridae) commensal with a sea urchin. Pacific Science 24: pp 90-98
McEdward, Larry R. and Benjamin G. Miner, 2006. Estimation and interpretation of egg provisioning in marine invertebrates. Integrative and Comparative Biology 46:3 pp 224-232
Miller, B.A. and R.B. Emlet, 1997. Influence of nearshore hydrodynamics on larval abundance and settlement of Strongylocentrotus franciscanus and S. purpuratus in the Oregon upwelling zone. Marine Ecology Progress Series 148: 83-94
Moberg, P.E. and R.S. Burton, 2000. Genetic heterogeneity among adult and recruit red sea urchins, Strongylocentrotus franciscanus. Marine Biology 136:5 pp 773-784
Nishizaki, Michael T. and Josef Daniel Ackerman, 2005. A secondary chemical cue facilitates juvenile-adult postsettlement associations in red sea urchins. Limnology and Oceanography 50(1): 354-362
Quinn, James F., Stephen R. Wing, and Louis W. Botsford, 1993. Harvest refugia in marine invertebrate fisheries: models and applications to the red sea urchin, Strongylocentrotus franciscanus. American Zoologist 33: pp. 537-550
Rogers-Bennett, Laura, Donald W. Rogers, William A. Bennett, and Thomas A. Ebert, 2003. Modeling red sea urchin (Strongylocentrotus franciscanus) growth using six growth functions. U.S. Fishery Bulletin 101(3): 614-626
Rogers-Bennet, L., H.C. Fastenau, and C.M. Dewees, 1998. Recovery of red sea urchin beds following experimental harvest. pp. 805-809 in Rich Mooi and Malcolm Telford (eds), Echinoderms: San Francisco. Proceedings of the Ninth International Echinoderm Conference, San Francisco, California USA 5-9 August 1996.
Schroeter, S.C., J.D. Dixon, T.A. Ebert, and J.V. Rankin, 1996. Effects of kelp forests Macrocystis pyrifera on the larval distribution and settlement of red and purple sea urchins Strongylocentrotus franciscanus and S. purpuratus. Marine Ecology Progress Series 133: 125-134
General Notes and Observations: Locations, abundances, unusual behaviors:
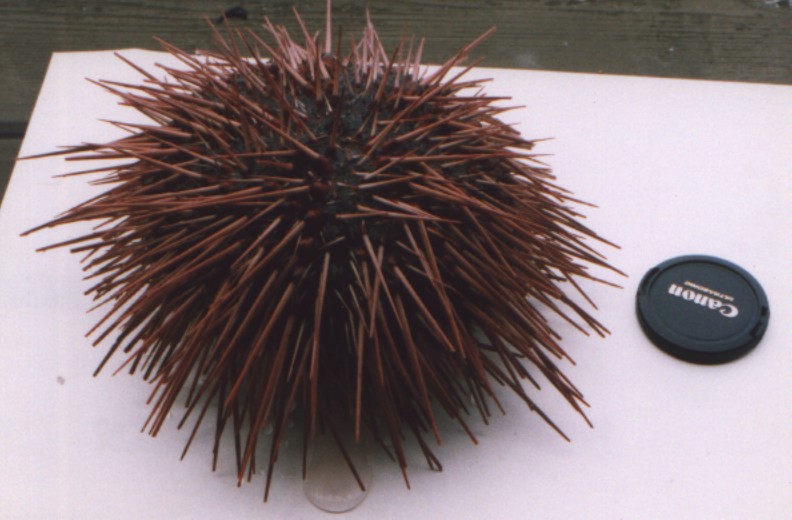
Another individual, collected off Sares Head July 1997. Photo
by Dave Cowles

As with all true urchins, the mouth (Aristotle's lantern, with 5
movable
jaws, each of which has a movable tooth) is in the center of the
underside
(oral side)
Photo by Dave Cowles, July 1997

This urchin was accidentally broken open. Inside one can see
the aristotle's lantern apparatus at bottom center around the mouth,
the
orange eggs clustered in the gonads on the upper side, the pinkish gut
spiraling around inside the test, and lines of diaphanous gray ampullae
(bases of the tube feet) lining the inside of the ambulacral region on
the test
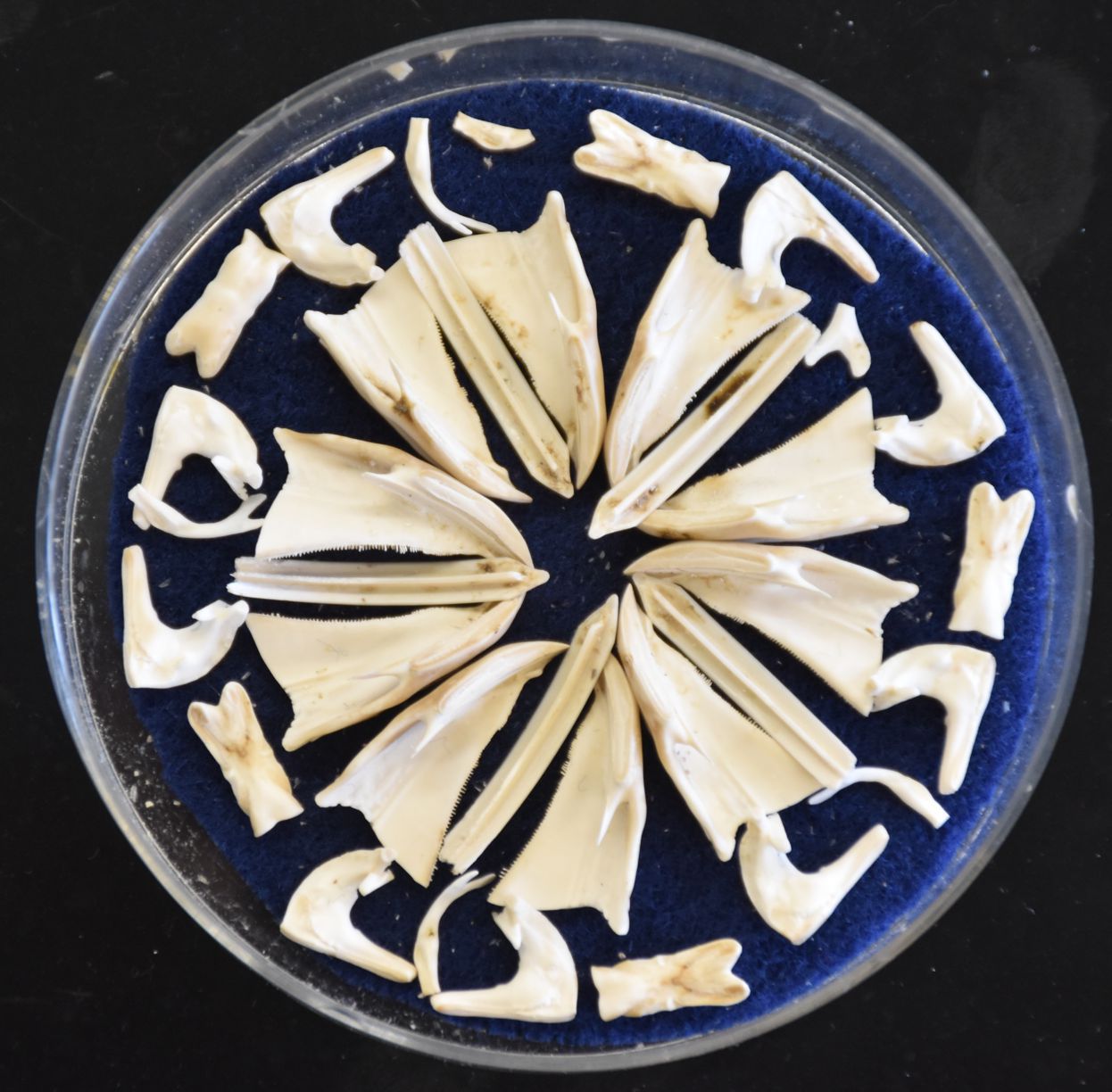
This photo shows the disassembled parts of an Aristotle's lantern from this species. A few of the pieces are missing. Photo by Dave Cowles, July 2020
As with all urchins, the internal ossicles are sutured together into a united shell-like structure called a test. Photo by Dave Cowles, July 2020
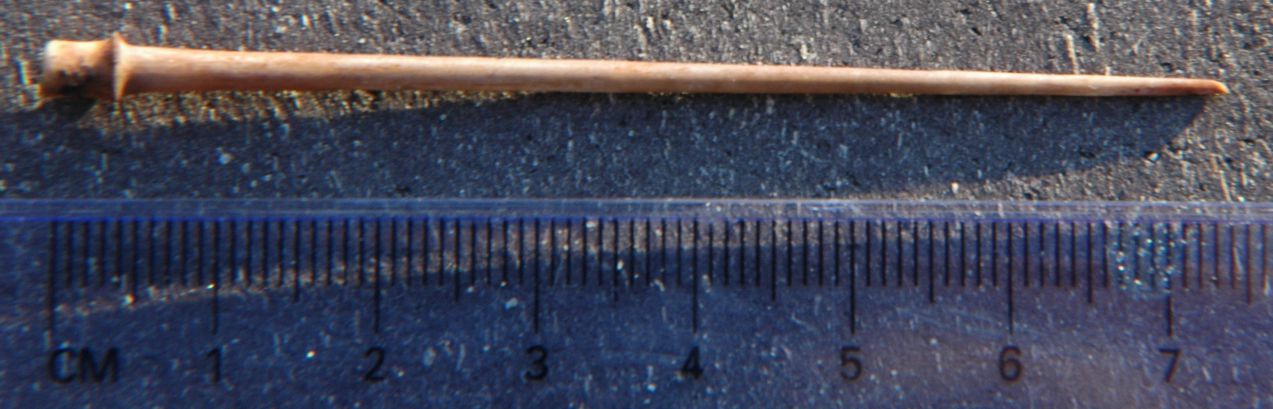
S. franciscanus spines are long, taper gradually,
and are much
larger than those of other local species. Contrast these with
spines
of S.
purpuratus, which are only about 2 cm or less long.

In this night photo, the fecal strands of the symbiotic amphipod Dulichia
rhabdoplastis can be seen streaming from the spine tips.
Underwater, night photo by Jim Nestler, July 2005

Another individual with Dulichia
rhabdoplastis, from 13 m depth at Coffin Rocks.
2014 photo by Dave Cowles
Authors and Editors of Page:
Dave Cowles (2005): Created original page




